Report: ‘On the Horizon’
Actionable insights into innovative medicines and their costs
March 2024 edition
The purpose of the ‘On the Horizon’ report
The NHCI released the 13th edition of the Horizonscan medicines database on December 5th, 2023. Since 2018, it has been offering a comprehensive view of upcoming medications in the Dutch market within the next two years. The database covers various types of drugs, including new proprietary ones, expanded indications for existing drugs, generics, and biosimilars. It provides detailed information on their mechanism of action, expected patient numbers, efficacy, registration dates, and financial impact. This data is crucial for decisions regarding medication inclusion in healthcare packages and pre-registration compensation negotiations. Additionally, the Horizonscan serves as an early information source for patients, healthcare professionals, hospitals, insurers, and government entities, helping them keep up with innovative medication advancements. With the pharmaceutical industry constantly changing, staying informed about new medications is challenging, making the Horizonscan a valuable tool for navigating this dynamic environment.
Actionable insights, better connected
The LOGEX Life Science team analysed the datasets of all Horizonscans of the past three and a half years (8 editions) and shared their actionable insights in the On the Horizon report, which can be downloaded on this page. It covers topics such as the number of new medicines per disease domain, the annual per-hospital budget impact of the most expensive upcoming medicines, such as Alzheimer’s and ophthalmological medicines, and the mapping of pharmaceutical interest in specific disease areas and types of medicines.

In the ever-evolving pharmaceutical landscape, keeping pace with the influx of new medications, particularly in high-cost disease areas like ophthalmology and Alzheimer’s disease, is daunting. Fortunately, the On the Horizon report provides a valuable summary for healthcare stakeholders as they navigate this complex environment.
Jan van der Eijk, Principle Real World Data LOGEX
Key insights
- Over the next two years, the majority of new medicines are anticipated to emerge in the areas of oncology, hematology, and neurology.
- Eye disorders such as Age-related Macular Degeneration (AMD) and Diabetic Macular Edema (DME) are contributing substantially to the rise in annual healthcare expenses. This increase is primarily attributed to the recent advancements in targeted treatments, which have the potential to extend the intervals between required injections, providing important patient- and healthcare resource utilisation benefits.
- Alzheimer’s medications are expected to have the biggest effect on hospital budgets. This is due to two new and expensive treatments on the horizon, adding up to over one billion euros in total costs.
- Significant healthcare expenditures are anticipated for treatments designed for Non-Alcoholic Fatty Liver Disease (NAFLD), with several drugs in the pipeline poised for introduction.
- The field of lung cancer is expected to experience a notable increase in the introduction of new medicines, including PD-1/PD-L1 inhibitors, tyrosine kinase inhibitors, and antibody-drug conjugates.
Expected annual budget impact of the most expensive drugs with registrations in the coming 2 years
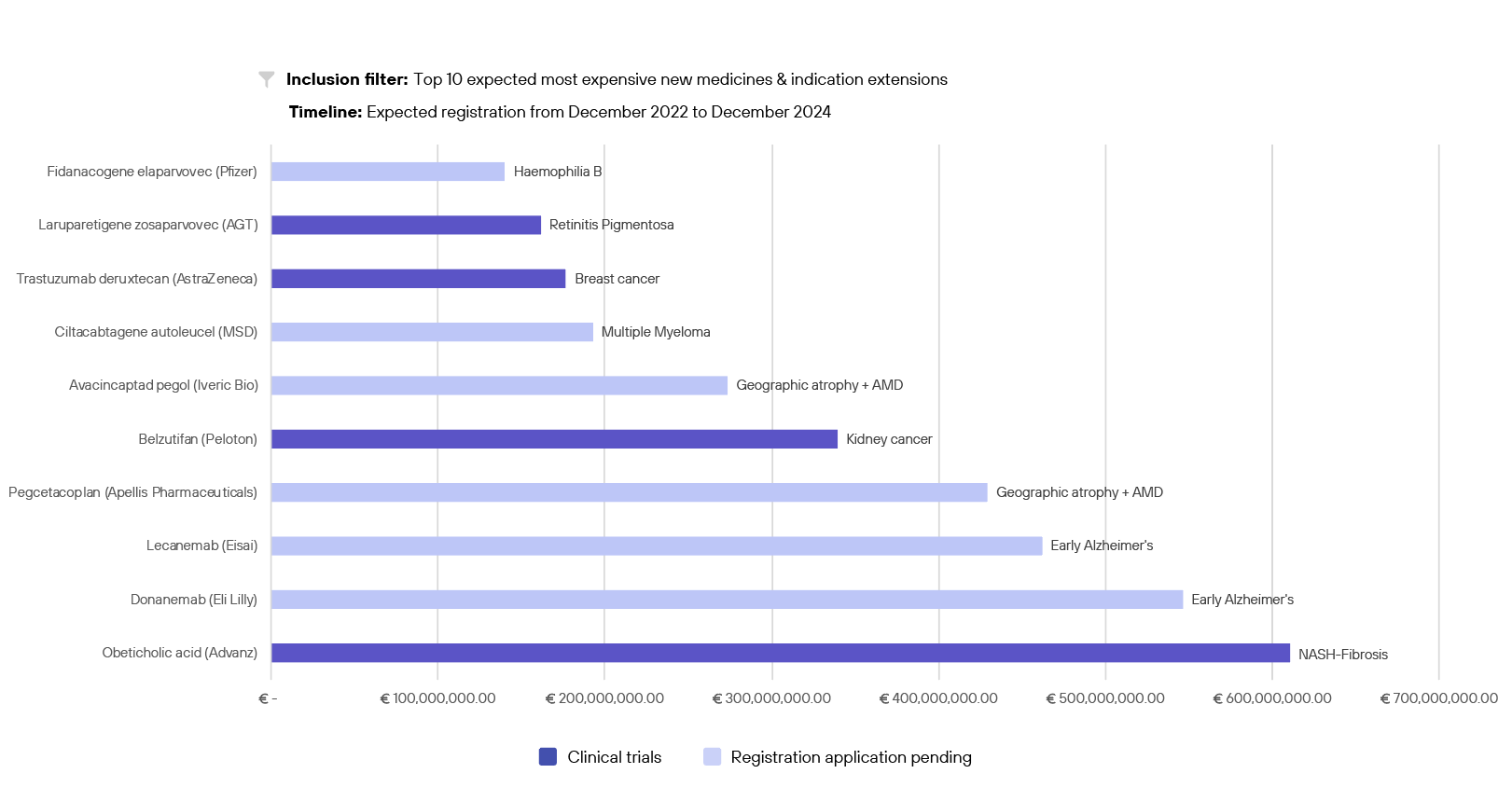
As healthcare expenditure continues to rise at a rapid pace, ensuring solidarity within our reimbursement system both now and in the future becomes increasingly crucial. This necessitates efforts to curtail drug prices and expenditure. While medications may offer health benefits, their associated prices may not always align proportionately, potentially compromising their effectiveness in delivering care. The importance of making prudent decisions regarding the allocation of collective financial resources is highlighted by the often-discussed comprehensive care agreement (Integraal Zorgakkoord).
In line with this, we strongly advocate for the use of real-world evidence to inform decision-making and enhance access to innovative treatments. Real-world evidence can play a pivotal role in monitoring health outcomes and cost benefits, complementing traditional clinical guidelines. By leveraging real-world data, we have the opportunity to develop real-world and real-time guidelines that can improve healthcare outcomes, whilst keeping the healthcare system affordable as a whole. If you’re interested in exploring how real-world evidence can enhance healthcare or would like more information about our projects across Europe, we encourage you to reach out to us to discuss potential collaborations.
‘On the Horizon’ report
Discover how Real-World Data can be used to monitor the impact of innovative medicines and how these insights can improve patient treatments.
On the Horizon report
Please fill in the form below to download the report.

