A robust platform that converts different types of hospital and patient data into real-world evidence
We prepare and pre-validate data and harmonise them to a common data model with a low burden for hospitals.
Join once…
Legal matters and data collection should not be part of individual treatment improvement projects. They should be done once and for all, giving back time and pace to the projects. ARWEN makes this a reality.
THE ARWEN DATA PROCESSING AGREEMENT
Our legal framework guarantee hospitals keep full ownership of their data. This eliminates the need to go through this legal process with each new project.
…benefit continuously
Particpants don’t need to go through data setup processes for each new project.
Join more meaningful treatment improvement projects faster.
1
WE SUPPORT BETTER HEALTHCARE WITH DATA ANALYTICS
We prepare, validate, anonymise and harmonise data from multiple disease groups to a common model for efficient analysis of the data. This allows us to deliver ready-to-use Real-World Evidence, with a minimal burden for your hospital.
ARWEN members can elect to participate in treatment improvement projects, while we support them in answering their own questions through data analytics.
We have 15+ years of experience with hospital data. Our scalable data models bring together patient pathway mapping, medicines uptake, clinical & patient outcomes, activity-based costs and more.
2
OUR PROJECT PROTOCOL ARE SHAPED IN COOPERATION
We propose a continuously evolving selection of focal therapeutic areas. We connect ARWEN partners from the healthcare and life science worlds who want to answer key questions and stimulate informed decision-making.
For each treatment improvement project, we involve suitable groups of healthcare providers that want to participate, based on their hospital profiles and therapeutic focus. This way, we ensure the projects lead to meaningful results that are relevant for everyone involved.
3
INVOLVED HOSPITALS HAVE ACCESS TO ALL RELEVANT INSIGHTS THAT COME OUT OF THE TREATMENT IMPROVEMENT PROJECTS
In ARWEN projects, project outcomes are shared with all the members involved. This makes your participation in ARWEN more rewarding.
What real-world data is supported?
OUR CORE DATA SETS
The core data sets represent the backbone of every ARWEN analytics project. They allow us to identify and structure the granular medical activities, care pathways, medicines prescriptions, clinical outcomes and more.
Claims and care activity data
This essential data set includes patient-level granular data on the single activities performed to treat patients, such as operations, hospitalisation time, diagnostics, consultations, etc.
Clustering and sequencing these activities allow, for instance, to generate of real-world patient pathways.
Prescription data
Pharmacy data provides detail on the types, dosage and volumes of drugs prescribed.
CROs
Clinician-Reported Outcomes include different types of quality indicators, such as reoperation rates, complications, time to next treatment, etc.
ADD-ON DATA SETS
These data sets are optional layers that can enhance the core ones. They can be utilised for specific study protocols and typically require advanced data models.
PROs
Patient Reported Outcomes refer to condition-specific indicators on the patient physical and mental status.
Pathology & lab data
While the occurrence of specific pathology & lab tests is included in the care activity data, this data sets discloses details on the results of pathology exams, such as blood tests, urinalysis, molecular pathology and more.
Other diagnostics data
While the occurrence of specific diagnostics is included in the care activity data, this data sets gives clarity on the results of additional diagnostic exams, such as X-rays, MRIs, CT scans and more.
Genomics data
DNA data can be crossed with other data sets to enable advanced insights on gene-based patient segmentation and support the discovery and development of precision therapy practices and medicines.
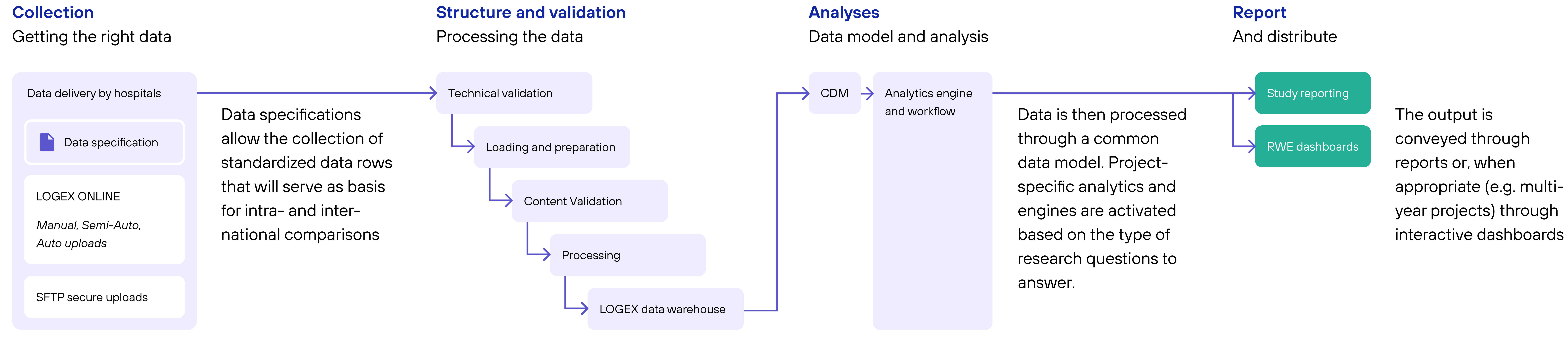
At LOGEX, we are expert data handlers and analysists…
- We have structured and routinised tools and practices for data extraction, linkage and validation
- During the process of validation, data is thoroughly reviewed ensure its validity and prepare it for use
- For all LOGEX client hospitals, they are already validated, structured and processed, allowing to limit effort and cut execution times
… continuously working to bring you more data sources.
- We have experience across a wide range of data-sets, from clinical registries to patient-level financial data
- Each study will be based on a set of core data set and, on optional add-on data sets
Who owns the data in ARWEN
Data governance is really important for us: data will always belong to hospitals.
Your data is yours
You are not our product, and neither is your data.
We are data analysts, not data broker
Your data is safe
The privacy and security of your data always comes first.
We hold ourselves to the same standards as you do
Your data is valuable
How you unlock the value in your data, is your choice.
We work to turn data into value, in full transparency
Your data is vital
Your data holds the potential to fast-track crucial research.
We are proud to be a highly respected & trusted third party
How does the ARWEN data model work?
- Differently from clinical trials, real-world data is not perfect and presents large variations across providers, regions and countries.
- As ARWEN is committed to build a comparable European data network, robust data models and policies must be established.
- Well-designed data specifications, a thorough validation process and smart algorithms utilised by ARWEN can turn the complexity of real-world data into tangible insights.