How RWE can improve all stages of the drug lifecycle
Introduction
In recent years, there has been an increasing focus on the cost of medicines. More and more new medicines seem to have a price tag that exceeds the general expectation of what is acceptable.
Pharmaceutical companies argue that the price is necessary to cover the developing costs of the medicine. People that are aware of all the aspects that go into developing and launching new medicines generally agree that it is indeed an extremely costly process. However, this does not mean there aren’t ways to increase the efficiency of this process. In fact, I believe Real-World Evidence (RWE), which refers to data and insights that are derived from real-world-settings, such as electronic health records (EHRs), claims data, patient-generated data, and other sources outside of clinical trials, has the potential to play a crucial role in this.
Pre-launch stages
The first stage of the drug lifecycle is discovery and development. It is important to note that life science companies invest millions in the discovery and development stage of many different treatments. On average only 1 or 2 out of 10,000 substances synthesised in laboratories and a mere 10% of drugs that make it all the way to the clinical trial phase actually end up as a marketable medicine, due to lack of efficacy, risks associated with the drug, or other compelling reasons. This means that there is no compensation for the pre-launch stages for most of the projects pharmaceutical companies invest in. It is often stated that the drugs that do make it to the market, have to earn back not only their Research and Development (R&D) costs but also those of failed projects.
There are many practical and regulatory reasons why the discovery process is the way it currently is. There is virtually no use of any Real-World Data in this phase of the drug lifecycle and I am aware that that fact is not likely to change in the foreseeable future. Still, if I am allowed to dream a bit, I would be able to imagine how RWE could help life science companies in this discovery phase. I invite you to dream along with me.
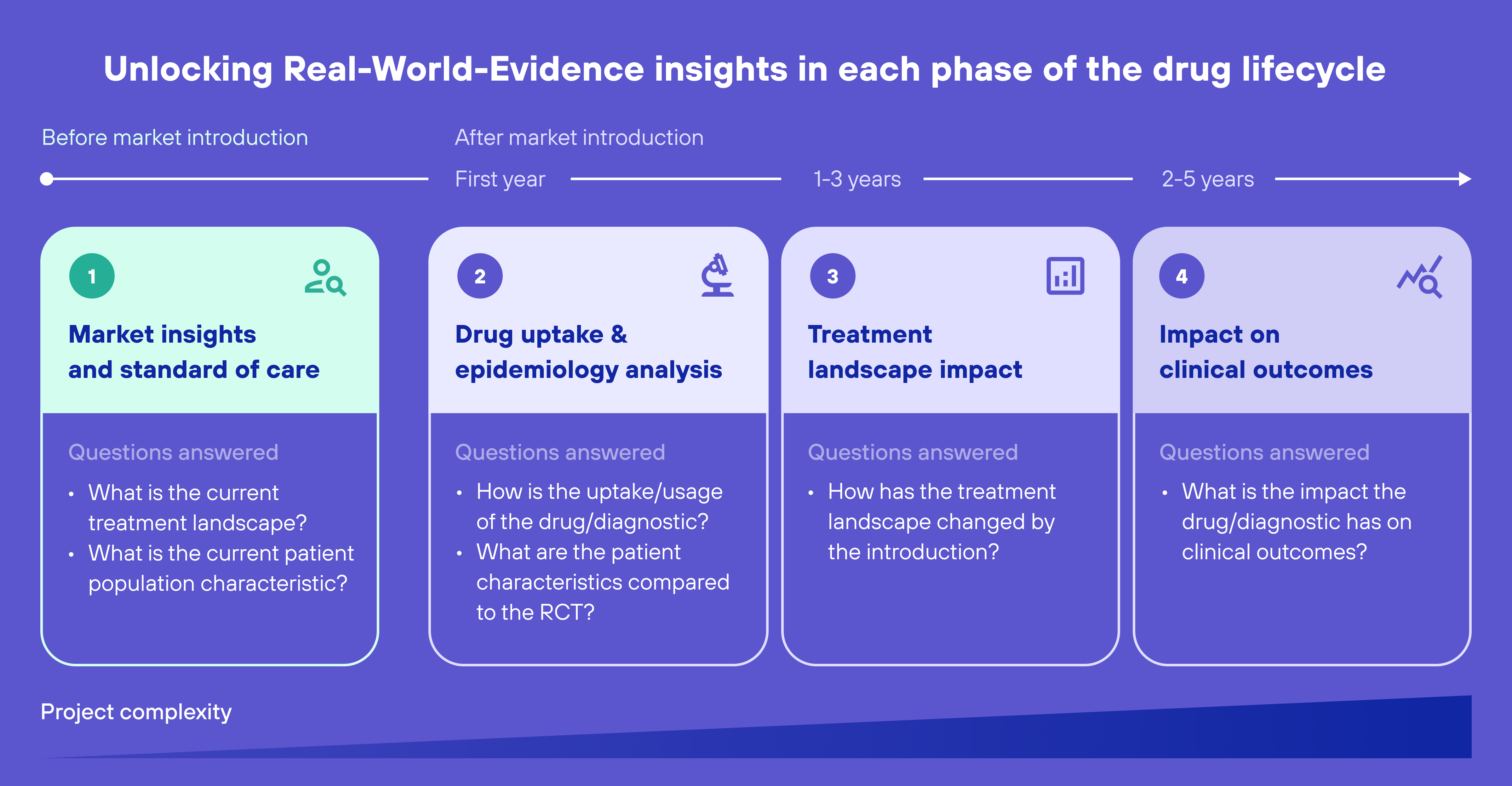
RWE could help to reliably outline the current market landscape and assess the standard of care within certain disease areas. By doing this, the following questions can be answered in the most accurate way:
- How are patients within a specific disease area currently being treated in the real world?
- How effective are those treatments on real-world patients?
This information could then be used to understand which patient groups lack good treatment options and identify other unmet needs of patients. RWE can also help to fill in evidence gaps about the level of efficacy of existing treatments. In an ideal world, these insights would lead pharma companies to focus exclusively on investing in better treatments for patients that are currently not receiving optimal care because of the existing issues.
After the discovery & development stage, there is a lengthy process of preclinical testing, clinical trials, and regulatory review. These stages are designed to evaluate the safety and efficacy of a new therapy in a controlled setting. Clinical trials are the gold standard and irreplaceable by RWE. However, it must be stated that they have some important limitations. For example, they are often conducted on a small and selective population of patients, which may not reflect the diversity of the therapy usage in the real world. Additionally, clinical trials are typically conducted over a short period of time and may not capture the long-term effects of the therapy. To predict how impactful these limitations are likely to be, it could be of value to dive into Real-World Data (RWD) and get answers to questions like:
- What are the current patient population characteristics?
- How deviant are these characteristics from those typically included in clinical trials?
I believe this analysis has the potential to be of great value when conducted early on during the drug discovery and development phase. If it were to reveal that it might be difficult to find participants for a clinical trial that not only closely resemble real patients, but also meet the safety criteria, this could lead to a decision to halt the development of the drug at that early stage. In other words, this RWD analysis holds the potential to help life science companies in making informed decisions about the feasibility of conducting meaningful and relevant clinical trials early on in the process.
Post-launch stage
When a new drug has succeeded to pass all pre-launch stages, it will be admitted to the market. After this, the post-launch stage starts. In this stage, it’s crucial to understand the therapy’s long-term safety and efficacy.
Shortly after the introduction of the therapy, RWE can supply data on how the drug is being prescribed and administered in real-world-setting, as well as monitor it for any potential safety concerns. At a later stage, data can be gathered to evaluate the therapy’s real-world effectiveness compared to other available treatments, and the drug’s impact on healthcare costs and patient outcomes. This could either show the life science company their drug is performing very well, or it could show new gaps the company might address with R&D.
Unlocking RWD for reliable RWE
Despite the exciting potential of RWE in supporting the therapies throughout the lifecycle, several challenges exist in the implementation. One of the biggest challenges is that data is scattered, not standardised, and not linked, making it difficult to extract the meaningful insights that form the sought-after RWE. Additionally, from recruitment to delivery, the process is time-consuming and complex. Hospitals are generally not properly involved or rewarded for contributing to RWE, and there are concerns about safety and privacy, as well as data ownership. These challenges are so severe that the use of RWE by life science companies is still in its infancy today.
Our Actionable Real World Evidence Network (ARWEN) aims to solve the challenges of RWE implementation by providing a robust platform that prepares, validates, harmonises, and analyses data from multiple disease groups to a common model for efficient analysis of the data. ARWEN’s goal is to connect forward-thinking hospitals, healthcare professionals, and clinicians who believe in making data-driven and evidence-based decisions when caring for patients. At the same time, the network makes it possible for life science companies to get the latest treatment insights to enable timely and better decision-making.
With our network of hospitals, we execute relevant treatment-improving RWE-projects like using RWE to tackle variations in treatments and outcomes in patients with breast cancer and metastatic hormone sensitive prostate cancer and getting valuable insights into medicine use for colorectal cancer through RWD. Visit arwen.eu to read more about ARWEN’s impact on improving data-driven decision-making processes in healthcare.
Author

Jan van der Eijk
Principal Life Science
jan.vandereijk@logex.com
Linkedin