Actionable RWE perspectives series
How can real-world evidence be used to solve unwarranted variation in drug uptake and clinical guidelines?
ARWEN RWE perspective report
Please fill in the form to immediately download the document
Questions addressed in this issue
Why is there variation in medicines uptake whilst clinical guidelines are in place?
How can we understand better what medicines are prescribed in the real world?
What can policymakers, physicians and life science companies learn from it?
How can we make sure this information is widely accepted, trusted and to make a tangible difference?
The background and rationale
The matter of drug uptake is raising great interest amongst European healthcare policymakers and the industry. The number of novel active substances, both in research pipelines and on the market, has grown exponentially over the past two decades. Also, with the use of new biomarkers and data sets, the ability to divide a patient population into distinct groups – each with specific needs, characteristics, and behaviours – is dramatically improving. Today, there are more medicines, for more and more specific patient segments than ever before.
Healthcare regulators seek to guide the prescribing behaviour of physicians, but with innovative therapeutic options, the need to adequately update and develop timely and accurate guidelines is increasingly challenging. One result of this is the large variation in the use of medicines within and across European countries. Proof of this statement has once again been delivered in last year’s International Medicines Uptake Comparator, carried out by LOGEX on behalf of NHS England. In this report, significant international drug uptake deviations and unwarranted variances from clinical guidelines in real practice were identified.
Medicines sales of selected breast cancer medicines across 5 European countries (IHE, 2018)
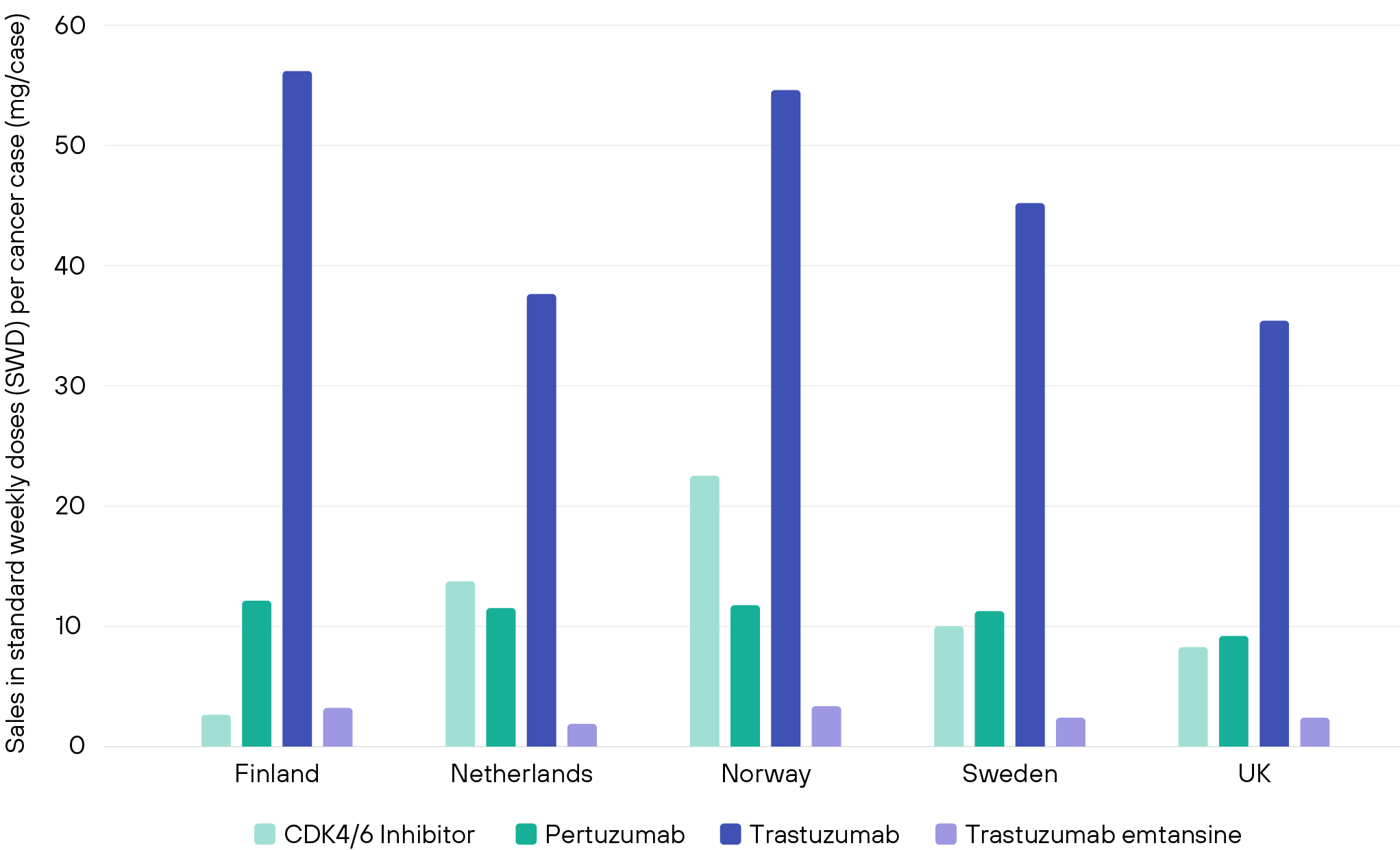
In healthcare systems where variation in treatments exists, variation in outcomes is an inevitable consequence. In this issue, we focus on how to address and reduce the lack of insights into variation in treatments and, consequently, in drug uptake.
Actionable insights, better connected
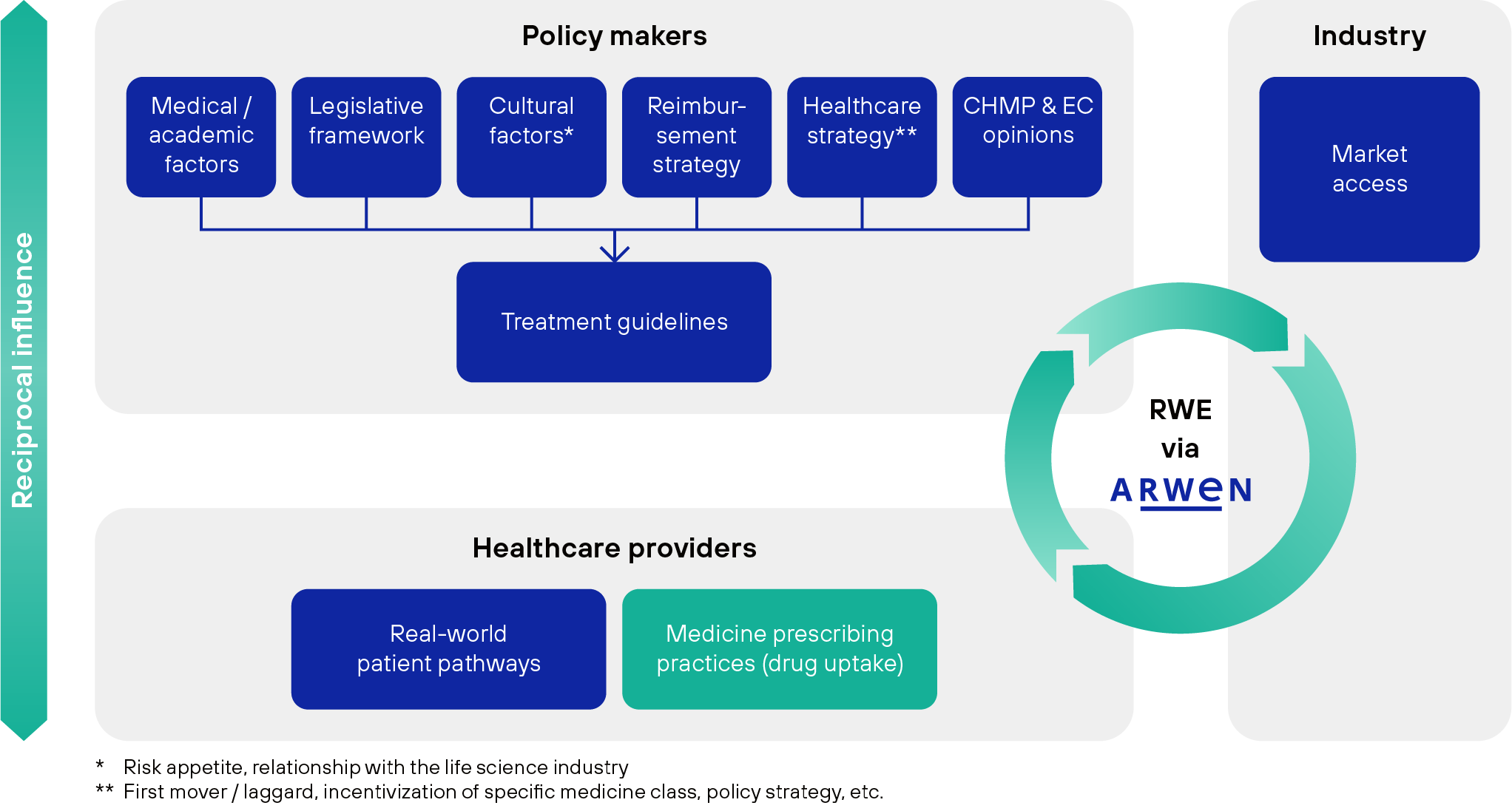
We believe Real-World Evidence (RWE) plays an essential role in both understanding and influencing the complex dynamics of drug uptake across countries. RWE can serve to invert the top-down approach, allowing policymakers and practitioners to learn about what procedures and medicines achieve the best results for determined patient classes, co-shaping policies with a fact-driven mentality.
Conceptual framework of drug uptake dynamics and correlations – real-world evidence as an enabler for establishing best practices in healthcare and creating reciprocal influence between policy makers, healthcare providers, and industry.
Read the full paper
To sharpen our hypotheses of the role that RWE can play in improving treatment guidelines and clinical outcomes in general, in this paper, breast cancer treatments are analysed and observations are shared from LOGEX’s work where we have integrated treatment indicators, guidelines and other patient data with standard uptake measurements.
Are you interested in knowing more about the role that RWE could play in reducing unwarranted treatment variation across countries? Download the report, it’s free.
ARWEN RWE perspective report
Please fill in the form to immediately download the document
About the authors
About the ARWEN RWE perspectives
This series is meant to bring you snippets of our latest ideas, reflections and opinions on the real-world evidence world. Our goal is to show innovative angles to interpret our healthcare systems and their future, stimulating thinking and further investigation. The series collects organic perspectives from our ARWEN experts, and it is not meant to be a scientific publication. All statements represent the opinions of the authors.


